Investigation of GAS outbreak in LTCF 2016 - Resident Re
Emergency Epidemic Investigation Data Collections - Expedited Reviews
Appendix 2. Resident Record Extraction Form
Undetermined source, mode of transmission, and risk factors for an outbreak of group A Streptococcus among residents of a long term care facility - Chicago, Illinois, 2016
OMB: 0920-1011
Study ID #: _R
Appendix 2. Investigation of GAS outbreak in an Long Term Care Facility, 2016
Resident Record Extraction Form
Form Approved; OMB No. OMB No. 0920-1011
Exp. Date 03/31/2017
Person completing form ______________________ Date Completed: ____/____/____
Resident (check one): Case Control
If CONTROL, date of matched case’s GAS culture: ____/____/____
GAS TESTING RESULTS
Did resident have any cultures/tests positive for GAS?
Yes No
# |
Date obtained |
Site cultured |
a. |
____/_____/_______ |
Blood Pleural Skin/Wound: _________ Rapid strep Sputum Joint Other __________ Throat Central line/TPN Catheter |
b. |
____/_____/_______ |
Blood Pleural Skin/Wound: _________ Rapid strep Sputum Joint Other __________ Throat Central line/TPN Catheter |
c. |
____/_____/_______ |
Blood Pleural Skin/Wound: _________ Rapid strep Sputum Joint Other __________ Throat Central line/TPN Catheter |
d. |
____/_____/_______ |
Blood Pleural Skin/Wound: _________ Rapid strep Sputum Joint Other __________ Throat Central line/TPN Catheter |
e. |
____/_____/_______ |
Blood Pleural Skin/Wound: _________ Rapid strep Sputum Joint Other __________ Throat Central line/TPN Catheter |
f. |
____/_____/_______ |
Blood Pleural Skin/Wound: _________ Rapid strep Sputum Joint Other __________ Throat Central line/TPN Catheter |
B. RESIDENT BACKGROUND
2. Sex: Male Female 3. Age: __________ 4. Date of birth: ____/____/____
5a. Room history for 1 month prior to GAS culture (for case) or time of time match (for control):
Room # (floor/wing) |
Dates in room |
Type of room |
Roommate (dates) |
a. |
___/___/____ to ___/___/____ |
Private Double Triple |
___/___/____ to ___/___/____ |
b. |
___/___/____ to ___/___/____ |
Private Double Triple |
___/___/____ to ___/___/____ |
c. |
___/___/____ to ___/___/____ |
Private Double Triple |
___/___/____ to ___/___/____ |
d. |
___/___/____ to ___/___/____ |
Private Double Triple |
___/___/____ to ___/___/____ |
e. |
___/___/____ to ___/___/____ |
Private Double Triple |
___/___/____ to ___/___/____ |
f. |
___/___/____ to ___/___/____ |
Private Double Triple |
___/___/____ to ___/___/____ |
5b. Did the resident have a roommate with GAS infection or colonization?
Yes No Unknown If yes: initials of GAS+ roommate__ Dates room shared: ___________
5c. Did the resident have frequent visitors during his stay in the facility? (if no, skip to 6)
Yes No Unknown
If yes: how many days per week?______ How many regular visitors/week?______________
6. Total length of stay at facility (most recent stay only) at time of GAS culture (mark only one):
≤ 1 week 1-3 weeks 4-8 weeks ≥ 8 weeks
7a. Is the resident deceased? Yes No If yes, date of death: ____/____/____
b. If resident died, death was: Related to GAS infection Possibly related to GAS infection
Not related Not applicable
8. Resident’s physicians?
Physician’s name |
Name of practice |
Specialty (e.g., wound care, etc.) |
a. |
|
|
b. |
|
|
c. |
|
|
d. |
|
|
9. List last admission prior to GAS infection or time of match for controls (including home, facility, hospitals, and any other LTCF).
Name & location |
Admission date |
Discharge date |
Diagnosis (if applicable) |
Admission from: |
a. |
______ / _______ / _______ |
______ / _______ / _______ |
|
|
b. |
______ / _______ / _______ |
______ / _______ / _______ |
|
|
C. MEDICAL HISTORY
10. Which medical condition(s) does the resident have? (mark ALL that apply):
Diabetes CHF/history of MI Peripheral vascular disease Stroke
Asthma/COPD Hypertension Chronic leg edema Recent herpes zoster
Dialysis Renal insufficiency Dementia Chronic skin condition
Cancer, specify type: _________________ Immunosuppressed/immunosuppression None
Cirrhosis Recent IV Drug Use Prosthetic Other: _______________________
(Note: immunosuppression includes: HIV/AIDS, chemo, radiation, immunosuppressive meds, including tacrolimus [Prograf], sirolimus [Rapamune], mycophenolate mofetil [Cellcept], high-dose or chronic steroids [prednisone, methylprednisone, hydrocortisone, dexamethasone] methotrexate.)
11. Weight: ____________ lbs or kg (circle unit of measure) 12b. Height: __________
12. Did patient have any surgical wounds, pressure ulcers, or other wounds at the time of admission to the facility?
Yes If yes, how many _____ No
13. Did patient have any surgical wounds, pressure ulcers, or other wounds at the time of first GAS isolation for case or at time-match for controls?
No Yes If yes, how many _____
Indicate location(s):
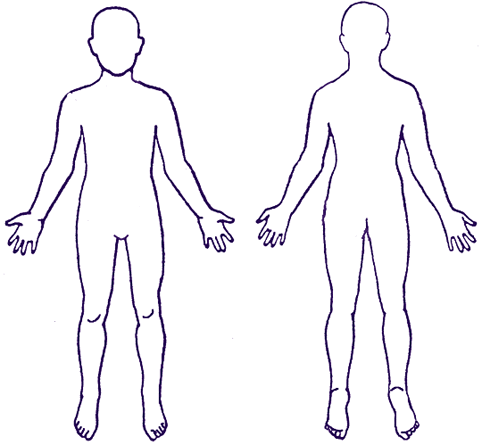 14.
Did the patient receive wound
care consultation
services within 1 month prior to the GAS case or time-match for
controls?
14.
Did the patient receive wound
care consultation
services within 1 month prior to the GAS case or time-match for
controls?
Yes No
Dates |
Name(s) of doctors or nurses |
|
|
|
|
|
|
|
|
15. Did the patient receive wound care WITHOUT wound care consultation within 1 month prior to GAS case or time-match for controls?
Yes No
16. Products used for wound care (surgical and nonsurgical) (check all):
Versafoam Granufoam Prisma Wound Matrix Mepilex Accuzyme
Ethyzyme DuoDerm Biotane Foam Hydrogel Wound vac
Antimicrobial cleanser/cream None Other: _____________________________
17. Has the patient had a surgical procedure within 1 month of GAS infection or time match for control?
Yes No
Procedure |
Date |
Incision Site |
|
______ / _______ / _______ |
|
|
______ / _______ / _______ |
|
18. Type of IV access present at time of positive GAS culture/referral from CC? None Not applicable
15a. Access Type |
15b. Date of Insertion |
15c. Person Inserting (e.g. RN) |
|
|
|
19. At time of GAS culture (case) or time-match (for control), was the resident diagnosed with:
Cellulitis Yes No Date of onset ___/___/____
Wound infection Yes No Date of onset ___/___/____
Pharyngitis Yes No Date of onset ___/___/____
Bacteremia Yes No Date of onset ___/___/____
Pneumonia Yes No Date of onset ___/___/____
Joint Infection Yes No Date of onset ___/___/____
20. Within 1 month of GAS culture or time-match for control, did the resident have any of the following signs or symptoms? (mark ALL that apply)
|
|
Date of onset (dd/mm/yy) |
|
a. |
Fever (≥100.5oF or 38oC) |
______ / _______ / _______ |
Max temp recorded: |
b. |
Sore throat |
______ / _______ / _______ |
|
d. |
Purulent discharge from wound |
______ / _______ / _______ |
Site: |
e. |
Wound – warm on touch |
______ / _______ / _______ |
Site: |
f. |
Wound – redness |
______ / _______ / _______ |
Site: |
g. |
Edema at the site |
______ / _______ / _______ |
Site: |
h. |
Increased pain at the site |
______ / _______ / _______ |
Site: |
i. |
Joint – warm on touch |
______ / _______ / _______ |
Site: |
j. |
Joint – redness |
______ / _______ / _______ |
Site: |
k. |
Joint – warm on touch |
______ / _______ / _______ |
Site: |
C. RESIDENT BASELINE STATUS (Can get further information from nursing)
21. Which appliances does the resident use (mark ALL that apply):
Tracheostomy Nasal cannula Oxygen mask Chronic Foley
G or J tube Nasogastric tube Colostomy/ileostomy Temporary Foley
Dialysis catheter PICC line Other, specify: ____________________________
22. Describe the resident’s ambulatory status: (mark ALL that apply)
Walks independently Walks with support Wheelchair Geri chair Bed bound
23. Indicate if resident incontinent of: (mark ALL that apply)
Stool Urine Not Incontinent Urinary catheter Colostomy/Ileostomy Unknown
24. Is the resident being tube fed? Yes No
25. Did the resident participate in the following activities in the 1 month prior to diagnosis or time-match for controls (mark ALL that apply):
a. PT/OT Times per 2 month period: ______
b. Speech pathology Times per 2 month period: ______
c. Podiatry Times per 2 month period: ______
d. Other: ____________________ Times per 2 month period: ______
Public reporting burden of this collection of information is estimated to average 45 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information including suggestions for reducing this burden to CDC/ATSDR Reports Clearance Officer; 1600 Clifton Road NE, MS D-74 Atlanta, Georgia 30333; ATTN: PRA (0920-1011)
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Group A Strep Investigation - 2003 |
| Author | EPO |
| File Modified | 0000-00-00 |
| File Created | 2021-01-23 |
© 2026 OMB.report | Privacy Policy